U. S. News
BioNTech to seek approval to give COVID vaccine to 5-year-old children
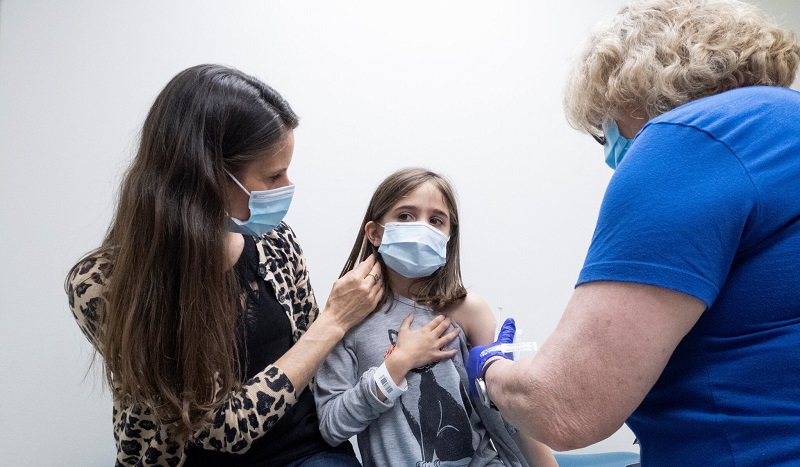
Rochester, New York – According to a report from Der Spiegel, vaccine maker BioNTech will request global approval to use its COVID-19 vaccine in children as young as five over the next few weeks.
The company, which partners with Pfizer said that it laid out plans to seek approval in children aged six months to two years old later this year, while on five to 11-year-olds to the end of September.
In the U.S., the Los Angeles board of education voted Thursday to require students 12 and older to be vaccinated against the coronavirus to attend in-person classes.
By now, there’s no such requirement in place in New York.
The Pfizer-BioNTech became the nation’s first vaccine to move beyond Emergency Use Authorization (EUA). It’s vaccine received full approval on Aug 23.
-

 Local News1 week ago
Local News1 week agoFriends and family assemble to honor the victim of the Driving Park Bridge hit-and-run
-

 New York1 week ago
New York1 week agoDue to the bird flu, New York has added new, temporary regulations for the import of dairy cattle
-

 Local News2 weeks ago
Local News2 weeks agoStrong Hospital has a significant police presence during a search for a man from Livingston County
-

 Local News1 day ago
Local News1 day agoThe people of Rochester come together to honor Reverend Iris J. Banister’s life and legacy
-

 Local News2 weeks ago
Local News2 weeks ago22 exotic animals left behind in a downtown Rochester building following the eviction of the tenant
-

 Local News2 weeks ago
Local News2 weeks agoBy April 29, RCSD intends to name an interim superintendent
-

 Local News2 weeks ago
Local News2 weeks agoWith the help of her kids, a Rochester teacher runs the Boston Marathon
-

 Local News1 week ago
Local News1 week agoThis weekend, there will be cleanup events in the Rochester region





